Free DAT Practice Questions
If you don’t have time for a full DAT practice test, and you need just a few sample DAT questions to see where you stand, you’ve come to the right place.
DAT Practice Questions
Practice Question #1: Biology
Which of the following illustrates the principle of induction in invertebrates?
- A. In an embryo, the presence of a notochord beneath the ectoderm results in the formation of a neural tube.
- B. A neuron synapses with another neuron via a neurotransmitter.
- C. Eye muscles constrict in response to light.
- D. Secretion of TSH stimulates the secretion of thyroxine.
- E. The maternal parent induces spontaneous expression of recessive genes.
Practice Question #2: Biology
C6H12O6 + O2 CO2 + H2O
This process is completed
- A. in the cytoplasm.
- B. in the area of the cell membrane.
- C. in the nucleus.
- D. in the mitochondria.
- E. in the area around the ribosomes.
Practice Question #3: Biology
Cells that are involved in active transport, such as cells of the intestinal epithelium, utilize large quantities of ATP. In such cells there are
- A. high levels of adenylate cyclase activity.
- B. many polyribosomes.
- C. many mitochondria.
- D. high levels of DNA synthesis.
- E. many lysosomes.
Practice Question #4: Biology
In an emergency, an individual with type AB antigen on his red blood cells
- A. may receive a transfusion of type O blood.
- B. may receive a transfusion of type A blood.
- C. may receive a transfusion of type B blood.
- D. All of the above
- E. None of the above
Practice Question #5: General Chemistry
The least stable free radical is
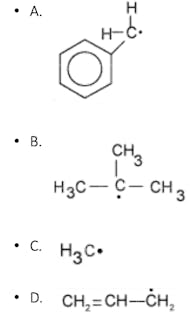
Practice Question #6: General Chemistry
Which one of the following reactions will be accompanied by an increase in entropy?
- A. Na(s) + H₂O(l) → NaOH(aq) + H₂(g)
- B. I₂(g) I₂(s)
- C. H₂SO₄(aq) + Ba(OH)₂(aq) → BaSO₄(s) + H₂O(l)
- D. H₂(g) + 1/2 O₂ (g) → H₂O(l)
- E. None of the above.
Practice Question #7: General Chemistry
The heat of combustion of gaseous ammonia, NH₃(g), is 81 kcal/mole. How much heat is released in the reaction of 34 grams of ammonia with excess oxygen?
- A. 40.5 kcal
- B. 60.3 kcal
- C. 75.8 kcal
- D. 81 kcal
- E. 162 kcal
Practice Question #8: General Chemistry
Which of the following electron configurations represents the element that would form the most acidic anhydride?
- A. 1s²2s²2p⁶3s¹
- B. 1s²2s²2p⁶3s²
- C. 1s²2s²2p⁶3s²3p¹
- D. 1s²2s²2p⁶3s²3p²
- E. 1s²2s²2p⁶3s²3p³
Practice Question #9: Math
A roulette wheel consists of 38 slots. 36 of these are numbered 1 through 36 and colored red or black so that there are nine red even-numbered slots, nine black odd-numbered slots, etc. These slots occur with equal probability. The two slots marked 0 or 00 are each three times as likely to occur as any one of the other 36. What is the probability that a red even number, a red 23, or a 00 will occur on one roll of the wheel?
- A. 2/7
- B. 5/18
- C. 13/38
- D. 13/42
- E. 3/10
Practice Question #10: Math
If 1/5 of those in a high school are freshmen, and 40% of the freshmen are women, and 3/5 of those in the other grades are women, how many men are enrolled in the high school, given that there are 600 students altogether?
- A. 240
- B. 264
- C. 300
- D. 336
- E. 360
Practice Question #11: Math
In the first 12 games of the season, the forward on a soccer team scored 20 goals. How many goals must he average in each of the remaining 6 games of the season in order to average 2.0 goals per game overall?
- A. 1 ½
- B. 2 ⅓
- C. 2 ½
- D. 2 ⅔
- E. 20
Practice Question #12: Math
Susan has $10 with which to buy apples. Apples are priced at 6 for $0.89, or $0.20 each. How much change will Susan receive if she buys as many as she can?
- A. $1.10
- B. $0.89
- C. $0.21
- D. $0.10
- E. $0.01
Practice Question #13: Keyholes
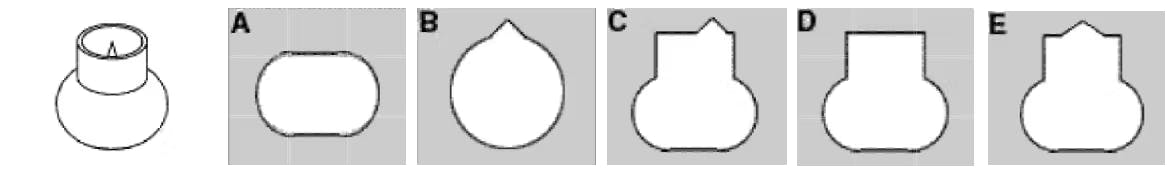
Practice Question #14: Angle Ranking
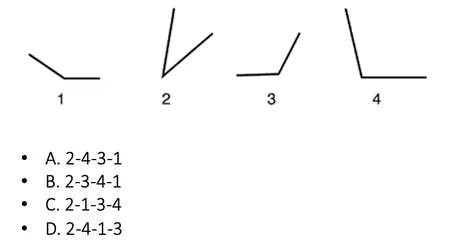
Practice Question #15: Hole Punching
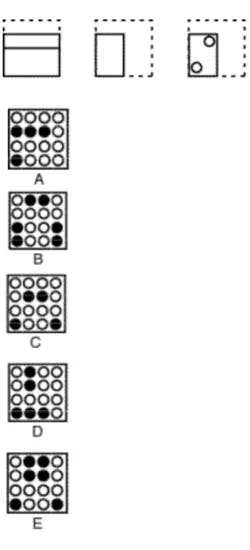
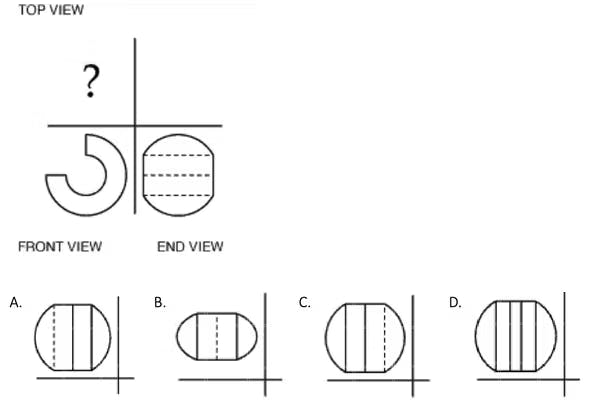
Practice Question #16: Top-Front-End
Ready to get started?
Let our expert teachers be your guide with a prep course that fits your schedule. No matter what stage of prep you’re in, Kaplan can help raise your score. †
